Pharmacy Prep Area
Are you affected by this deadline? Take the USP 800 Quiz to find out!
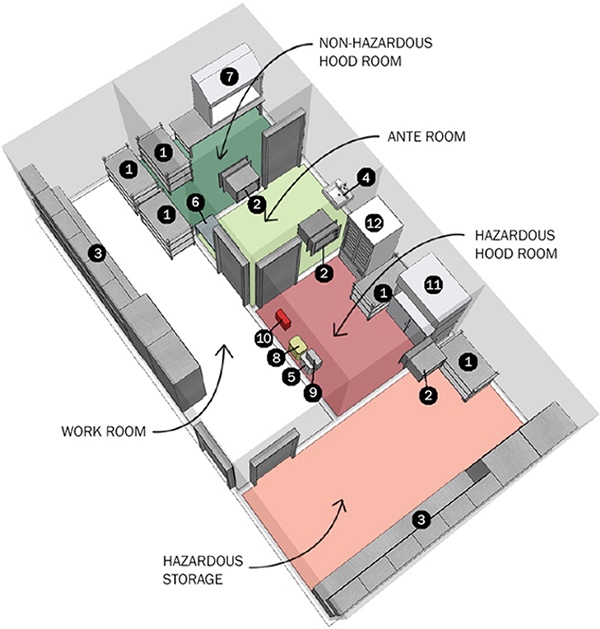
Pharmacy Prep Area Key and Room Pressurization Scale
On December 2019, facilities handling hazardous drugs must comply with the requirements of USP 800. Starting now will leave you time to secure funding, gather a team, construct the project, and most importantly, get it certified.
Are You Affected By This Deadline? Take the USP 800 Quiz
While the requirements are more specific than this, a couple questions can help identify if additional research is needed:
- Does your facility have any negative pressure hoods?
- Do you handle or store unpackaged hazardous drugs for durations longer than 12 hours?
If you answered “yes” to either of these questions, additional review of the USP 800 language is advised.
How Does USP 800 Affect Me?
The main goal of USP 800 is to provide standards regarding the safe handling of hazardous drugs to minimize the risk of exposure to patients and staff. The standard includes many processes to properly unpack, handle and store hazardous drugs, in negatively pressurized environments. This may require infrastructure changes in any or all of the following areas:
- Shipping and Receiving – Unpacking and transporting hazardous drugs should occur in areas other than sterile compounding or positive pressure areas.
- Storage – Hazardous drugs shall be stored separately from other inventory, in a negatively pressurized space (including refrigerated hazardous drugs in their own, dedicated refrigerator in the negative space).
- Cytotoxic Drugs – Processing of Cytotoxic drugs must be separate from other sterile preparation spaces and are to be exhausted directly to the building exterior, at a point 10’ above the roof or adjacent openings.
- Facility Design – Rooms shall be constructed to maintain pressurization and require finishes which facilitate cleaning and decontamination.
What’s next?
- Start with the short USP 800 Quiz.
- Talk to your pharmacist(s) to develop an action plan.
- Determine where your facility is impacted by the new USP 800 deadline.
- Contact us in the process of making existing facilities compliant with USP 800, from compliant floor coverings to increased airflow requirements.
More information on USP 800
